






Please visit my author page at amazon.com

The simplest definition of an electromagnetic wave is that it is the way in which certain kinds of energy (light, radio, microwaves, infrared, visible light, ultraviolet, x-rays, and gamma rays) travel through space. These are all examples of electromagnetic radiation.
In other words, when you see light, what is happening is that something (the sun, a star, a flame, etc.) is giving off electromagnetic waves. These waves travel through space until they hit your eye, which reacts to the waves, and you perceive this as light.
If electromagnetic waves are passing through a vacuum, then they travel at the speed of light (approximately 186,000 miles per second, or 300 million meters per second). If the waves travel through other objects, then they will travel at a lower speed. Some electromagnetic waves (such as visible light) are blocked by most solid objects. This is why you can't see through solid walls. But some electromagnetic waves, such as x-rays, gamma rays, and radio waves, are able to travel through some solid objects.
An electromagnetic wave gets its name from the fact that it is a combination of an electrical field and a magnetic field.
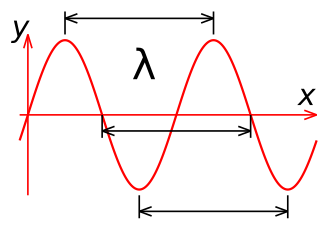
All waves have two properties called the frequency and wavelength. The intensity of the wave (which is called the amplitude) keeps reversing on a regular basis as it moves through space. If you were able to measure the intensity at a given point, it would go up and down at a certain rate. This rate is called the frequency, and it's measured in cycles per second. Another name for cycles per second is Hertz (Hz). For example, if it's a radio wave that's changing intensity a million times per second, we would say that this wave has a frequency of 1 million cycles per second, or 1 million Hertz, or 1 Megahertz (MHz).
The other important property of a wave is its wavelength. Since the wave is changing intensity on a regular basis as it moves through space, it is high at some points and low at other points. To measure the wavelength, you would find two points where the intensity is exactly the same. The distance between these two points is the wavelength. Wavelength is usually measured in meters. In the illustration at the left, the blue lines show the wavelength of the wave.
Frequency and wavelength are related, so if you know one, you can calculate the other. The frequency times the wavelength equals the speed of light (300,000,000 meters per second). For example, a radio wave changing a million times per second (1 MHz) has a wavelength of 300 meters. This is because 1,000,000 Hz times 300 meters equals 300 million meters per second.

The different kinds of electromagnetic waves are different because they have different frequencies and wavelengths. You can't see x-rays or radio waves because your eyes are only sensitive to a narrow band of frequencies. The different kinds of energy caused by different frequencies is called the
electromagnetic spectrum,
which is shown on the chart at the right. As you can see, the longest wavelengths (the lowest frequencies) are radio waves. As the wavelength gets smaller (the frequency gets higher), they become microwaves and then infrared light.
If the wavelength gets even shorter (the frequency gets higher), then the waves become visible light. As you can see from the chart, different colors of light have different frequencies and wavelengths. Red light has the longest wavelength and lowest frequency. Violet light has the shortest wavelength and highest frequency. In order of lowest frequency to highest frequency (longest wavelength to shortest wavelength), the colors are in this order: Red, Orange, Yellow, Green, Blue, Indigo, Violet. You can remember this by learning the name Roy G. Biv. Roy G. Biv is not a real person, but the name is just an easy way to remember the order in which the colors appear in the rainbow, and in the electromagnetic spectrum.
If the wavelength gets shorter (and the frequency gets higher), then the wave turns into ultraviolet light, then x-rays, and then gamma rays.
For hundreds of years, scientists realized that light and these other forms of energy consisted of waves. But in the 20th century, when quantum physicists like
Einstein looked at the universe, the wave explanation didn't match up with with some experimental results. So they came up with another explanation, that light and these other kinds of energy are made up of tiny particles called
photons. This new way of looking at light explained some phenomenon. But when looking at other phenomenon, the photon explanation didn't match up with the results of experiments.
Because of these contradictory results, scientists now simply say that there is a
"wave-particle duality". This is just a way of saying that for some purposes, light acts like a wave, but for other purposes it acts like a particle.
Please visit my blog for more great science fair ideas.
Images courtesy of Wikipedia
Please visit my author page at amazon.com